This chart is talking about the comparision of serum cortisol and plasma ACTH to known ranges for known times. Serum cortisol should be measured at 8 a.m., 4 p.m., and around midnight (most clinical studies use 11 p.m. - 1 a.m.). If you remember, this will show the diurnal variation (or lack thereof). If the diurnal rhythm is not normal, this is one clue for the diagnosis of Cushing's.
According to Esoterix Labs, normal adult ranges for serum cortisol are:
- 8:00 a.m. 8.0 – 19 ug/dL
- 4:00 p.m. 4.0 - 11 ug/dL
- midnight close to zero
In The Biochemical Investigation of Cushing Syndrome[Neurosurg Focus 16(4), 2004. © 2004 American Association of Neurological Surgeons], the last page says:
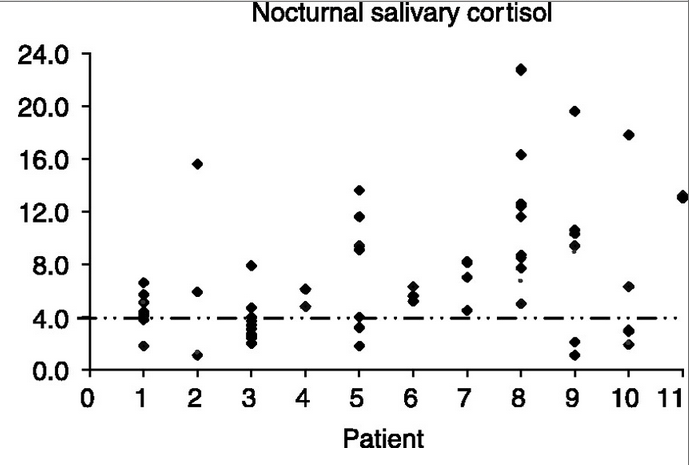
In patients with Cushing disease, 50% have a 9 a.m. plasma ACTH level within the normal reference range of 9 to 54 pg/ml (2–12 pmol/L) and the remaining patients have a slightly elevated ACTH level.[36] Due to the loss of circadian rhythm, however, nighttime ACTH secretion is abnormal. A midnight plasma ACTH levelgreater than 23 pg/dl (5 pmol/L) confirms the presence of an ACTH excess.Salivary cortisol tests are also done to determine diurnal/circadian rhythm. Since midnight serum cortisols are more difficult to do because the patient has to go to an open lab late at night, salivary kits offer a much easier alternative. However, serum cortisol tests tend to work better for cyclical patients because the serum level has to be pretty high before the cortisol is readable in saliva. Esoterix has developed a more sensitive assay for testing salivary cortisol which may offer a comparable output.
There are four FDA-approved labs for testing salivary cortisol (Quest, ACL Labs, Esoterix, and Labcorp), and each uses it's own method with varying ranges. The ranges for Esoterix are below:
24-hr Urinary Free Cortisol (UFC) is another test that is used. Again, depending on the method used to run these and the lab, the ranges can vary. I also discussed these in "When tests don't even rate an A+ or a C-" . These are used to get an average value of the excess cortisol secreted in a 24-hour period.
Some researchers also use a 10-hour UFC to see if excess is just secreted overnight. These are analyzed differently by looking at the cortisol/creatinine ratio. A ratio of 15 or higher is considered diagnostic.
There is some question about the validity of the dexamethasone suppression test, with various factions in the literature and in the research saying various things. Basically, it boils down to the dosage used with the dexamethasone and the doctor doing it.
An Update on the Overnight Dexamethasone Suppression Test for the Diagnosis of Cushing's Syndrome: Limitations in Patients with Mild and/or Episodic Hypercortisolism [T. C. Friedman, Exp Clin Endocrinol Diabetes. 2006 Jul;114(7):356-60] says:
The objective of this study was to determine the sensitivity of the one mg overnight dexamethasone suppression test in patients with mild and/or periodic Cushing's syndrome...DO NOT LET A DOCTOR TELL YOU THAT YOU DO NOT HAVE CUSHING'S BASED ON THE RESULT OF JUST ONE OR EVEN A FEW "NORMAL" TESTS!! In this post, I explained the difficulties in diagnosing cyclic/episodic/mild/subclinical Cushing's. Be prepared to do a lot of testing. If you are cyclic, you will have to figure out the symptoms of your "highs" versus your "lows". The only way to do that is to JOURNAL your symptoms and TEST! Keep very detailed records of all lab results. Make sure you get a copy of each. Compare the results to your symptoms. If you are consistent, you can figure out your cycle fairly quickly. In order to do this, you must find a doctor who is willing to let you test when you need to test.
Therefore, an overnight dexamethasone suppression test was performed in 17 consecutive patients presenting to an endocrinology clinic with signs and symptoms of hypercortisolemia who were later proven to have Cushing's syndrome...
[These patients] failed to suppress to a value less than this cut-off point (sensitivity of 41 %). These results demonstrate that the great majority of patients with mild and/or periodic Cushing's syndrome suppress to overnight dexamethasone. Since patients with mild and/or periodic Cushing's syndrome are the patients in whom the identification of hypercortisolism is difficult, our results from this relatively small study suggest that this test should no longer be used to exclude these patients from further workup for Cushing's syndrome.